
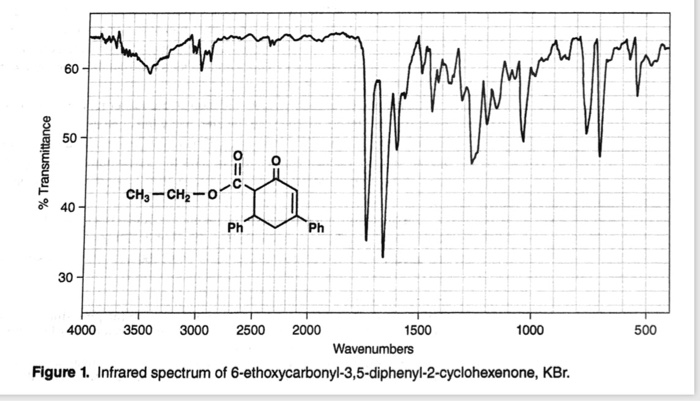
These peaks tend to appear as short, broad singlets. Protons directly attached to the alcohol oxygen often appear in the region of 2.0 to 2.5 ppm.The electronegativity of the alcohol oxygen de-shields these protons causing them to appear downfield when compared to alkane protons. Protons on carbon adjacent to the alcohol oxygen show up in the region of 3.4-4.5 ppm.In addition, the IR spectra will show the bands typical for aromatic compounds in the region of 1500-1600 cm -1. The IR spectrum of phenols the O-H stretch appears at roughly 3500 cm -1. The broadness of the O-H peak makes it very easy to distinguish in an IR spectrum. Instead of seeing one sharp peak, you see a broad set of multiple overlapping peaks. Because protons are shared to varying extent with neighboring oxygens, the covalent O-H bonds in a sample of alcohol all vibrate at slightly different frequencies and show up at slightly different positions in the IR spectrum. The rounded shape of most O-H stretching modes occurs because of hydrogen bonding between different hydroxy groups. Peak shapes are sometimes very useful in recognizing what kind of bond is present. Source: SDBSWeb : (National Institute of Advanced Industrial Science and Technology of Japan, 14 July 2008) In the IR spectra of 1-butanol, show below, the O-H stretch appears at 3300 cm -1 and the C-O stretch appears at 1073 cm -1.įigure IR8. In addition alcohol have a strong C-O stretch near 1000 cm -1. This exact position of the peak is dependent on the amount of hydrogen bonding in the alcohol. This peak tends to be very strong and very broad. The IR spectrum of aliphatic alcohols have a distinctive O-H stretch in the range of 3300 to 3400 cm -1.
The wavelengths from (approximately) 400 to 750 nanometers provide us with our physical view of the universe.\) Our eyes perceive a tiny sliver of the electromagnetic spectrum. To obtain a copy of this insightful report written in 2003, as well as other related articles, visit Werbach's website at See smart radio. Waves just mix together and become more difficult to differentiate, but modern electronics can, in fact, separate them. The old notion that radio waves interfere with and cancel each other is a false one. He states that many believe the traditional policy of dividing the airwaves into licensed bands now impedes progress because today's radio technologies allow for much more sharing of the spectrum than ever before. In Kevin Werbach's very educational white paper, "Radio Revolution," the author says an artificial scarcity has been created because policy makers do not understand the technology. There is a great deal of controversy over the licensing of frequencies. The spectrum can be viewed in meticulous detail from the Federal Communications Commission (FCC) and National Telecommunications and Information Administration (NTIA) by visiting and See electromagnetic radiation and wave.
See satellite frequency bands and optical bands.įrequencies above 40 GHz have not been licensed, but are expected to be made available in the future as the technology is developed to transmit at these smaller wavelengths (higher frequencies). Some frequency bands are used for the same purpose in all three regions while others differ. The radio spectrum, which includes both licensed and unlicensed frequencies up to 300 GHz has been defined worldwide in three regions: Europe and Northern Asia (Region 1) North and South America (Region 2), and Southern Asia and Australia (Region 3).
All rights reserved spectrumThe range of electromagnetic radiation (electromagnetic waves) in our known universe, which includes visible light. An Illustrated Dictionary of Aviation Copyright © 2005 by The McGraw-Hill Companies, Inc.


 0 kommentar(er)
0 kommentar(er)
